Eventually, these particular person legal guidelines have been mixed right into a single equation—the preferrred fuel law—that relates fuel portions for gases and is sort of correct for low pressures and average temperatures. We will take into account the important thing developments in particular person relationships , then put them mutually within the perfect fuel law. This is Avogadro's law, which states that beneath the identical temperature and pressure, equal volumes of all gases comprise the identical variety of molecules. This equation reveals that if the amount of fuel increases, the amount of fuel will boost proportionally. In different words, the variety of fuel atoms or molecules is unbiased of their sizes or the molar mass of the gas.
How To Find Pressure Of A Gas Given Temperature And Volume The legislation is understood as after an Italian scientist Amedeo Avogadro who revealed his speculation concerning the connection between the fuel quantity and its quantity in moles in 1811. The average fastened level of absolutely the temperature scale is called the triple level of water. The significance of this level might be seen in Figure 13, which reveals among the circumstances of temperature and strain underneath which a hard and fast quantity of water will exist as a good , a liquid or a fuel . Notice that the graph reveals low pressures, spherical 1% of average atmospheric pressure. But you too can see that the melting temperature, at which water makes the transition from good to liquid, will expand somewhat because the strain falls. As a consequence, the 2 strains meet at a temperature of 0.01 °C when the strain is 600 Pa.
Under these distinct situations water vapour, liquid water and reliable ice can all coexist in equilibrium. Avogadro's Number, the perfect fuel constant, and each Boyle's and Charles' legal guidelines mix to explain a theoretical best fuel wherein all particle collisions are completely equal. The legal guidelines come very near describing the conduct of most gases, however there are very tiny mathematical deviations because of variations in proper particle measurement and tiny intermolecular forces in proper gases. Nevertheless, these crucial legal guidelines are sometimes mixed into one equation generally recognized because the perfect fuel law.
Using this law, you will discover the worth of any of the opposite variables — pressure, volume, variety or temperature — in case you understand the worth of the opposite three. Boyle's regulation asserts that when the temperature of a hard and fast amount of fuel is held constant, the product of its strain and quantity is constant. Charles' regulation asserts that when the strain of a hard and fast amount of fuel is held constant, the amount is proportional to the amount (Tcen + 273° centigrade), the place Tcen is the centigrade temperature. This means that there exists a lowest a possibility temperature, in most cases referred to as absolute zero, at about −273° centigrade.
You can use values for proper gases as lengthy as they act like perfect gases. To use the formulation for an actual gas, it should be at low strain and low temperature. Increasing strain or temperature raises the kinetic power of the fuel and forces the molecules to interact. While the perfect fuel legislation can nonetheless provide an approximation beneath these conditions, it turns into much less correct when molecules are shut mutually and excited.
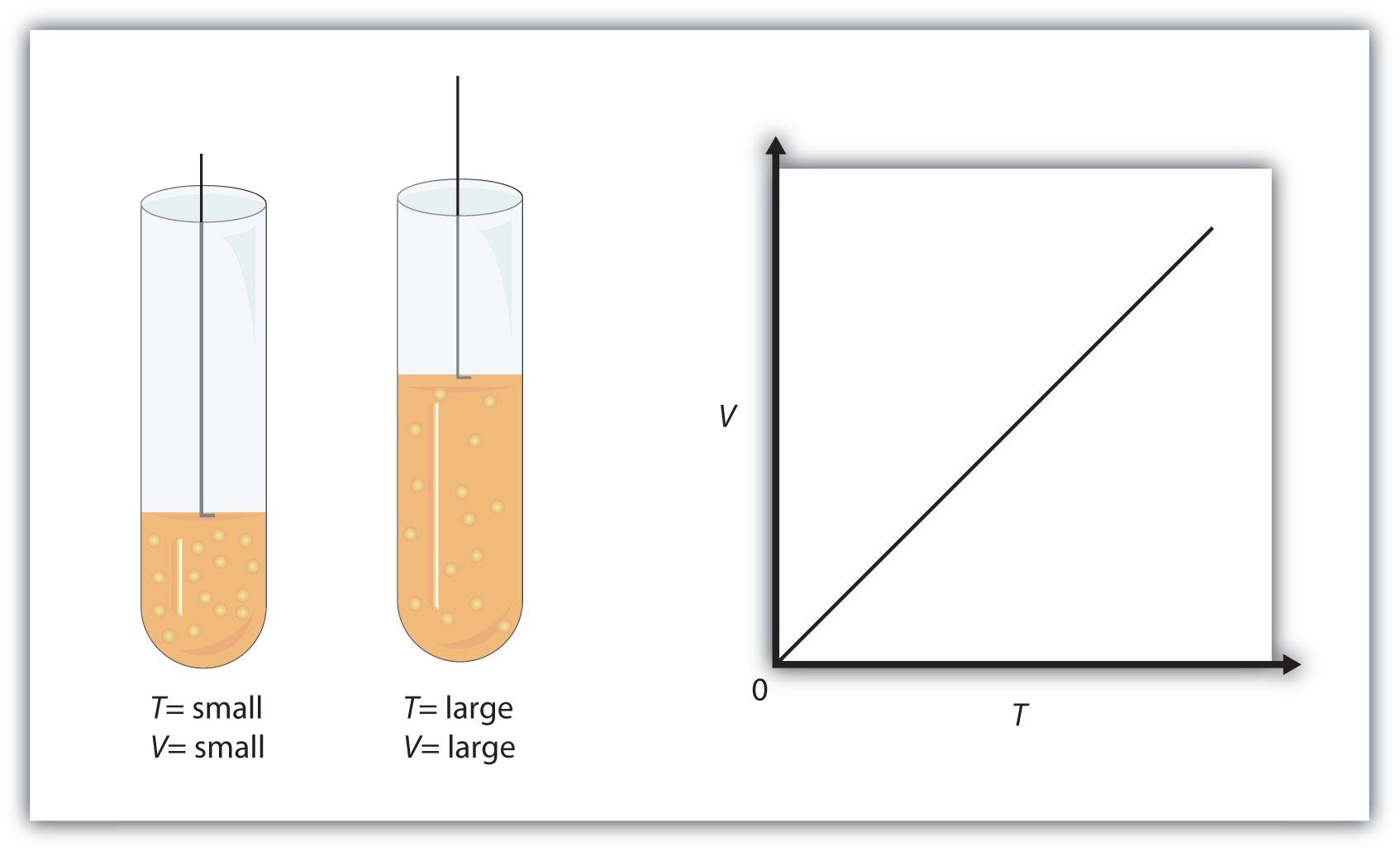
The conduct of gases may be described by a number of legal guidelines situated on experimental observations of their properties. The strain of a given quantity of fuel is immediately proportional to its absolute temperature, furnished that the quantity doesn't change (Amontons's law). The quantity of a given fuel pattern is immediately proportional to its absolute temperature at fixed strain (Charles's law).
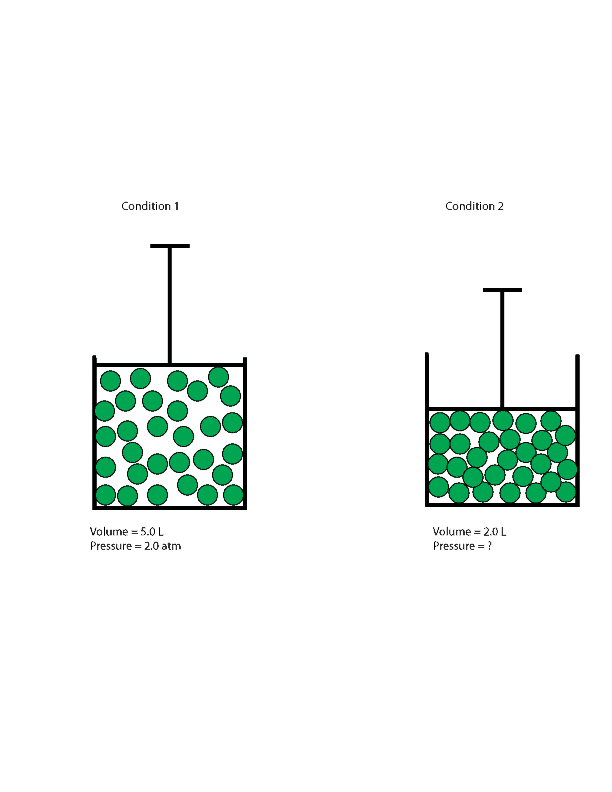
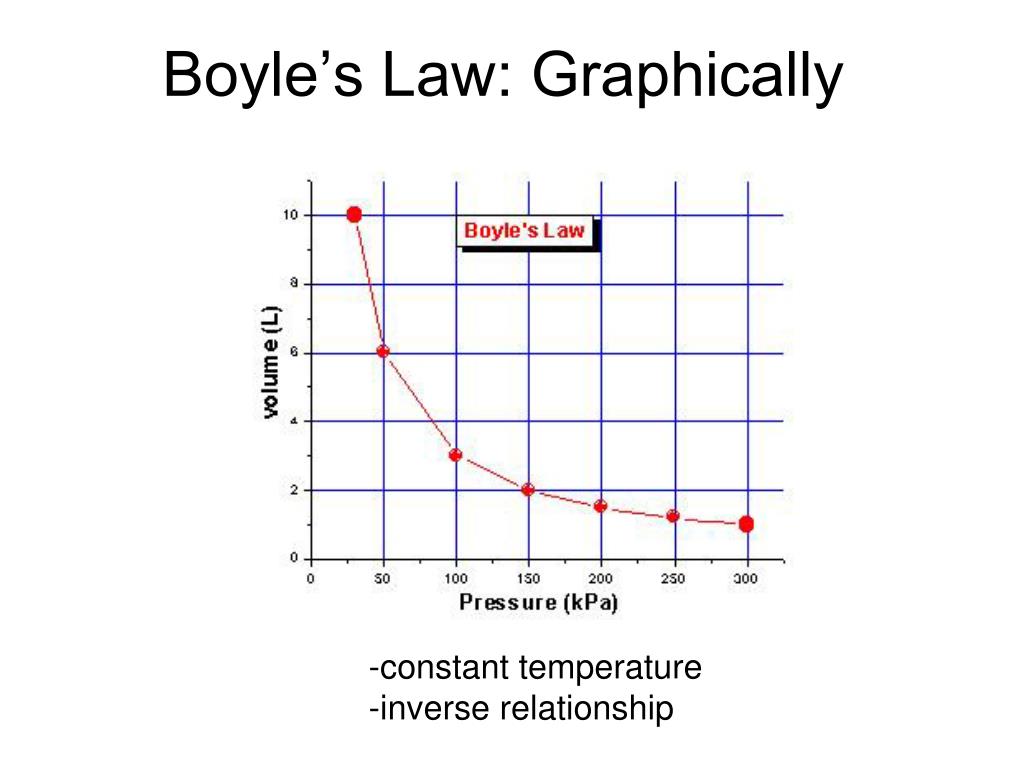
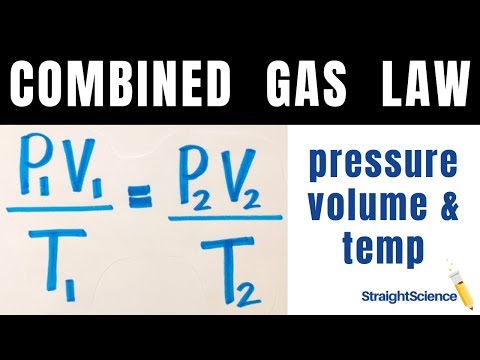
The quantity of a given quantity of fuel is inversely proportional to its strain when temperature is held fixed (Boyle's law). Under the identical situations of temperature and pressure, equal volumes of all gases comprise the identical variety of molecules (Avogadro's law). First, we have to decide the question, which is to calculate the quantity of a quantity of fuel at a given temperature and pressure. In a second step, after establishing a basis, we have to convert the mass of methane that would be the proposal into pound moles. Third, we have to convert temperature in levels Fahrenheit into absolute levels Rankin and, fourth, convert strain from psig into psia. Fifth, we have to pick out the suitable preferrred fuel fixed and use it with a rewritten kind of Equation 4.11 to work out the quantity of 11.0 lbs of methane gas.
Finally, we will substitute the values beforehand decided into the rewritten equation to calculate the volume. Gases whose properties of P, V, and T are precisely described by the perfect fuel regulation are pronounced to exhibit best conduct or to approximate the traits of a great gas. An best fuel is a hypothetical assemble that could be used together with kinetic molecular principle to efficiently clarify the fuel legal guidelines as will probably be described in a later module of this chapter. Although all of the calculations introduced on this module assume best behavior, this assumption is just affordable for gases underneath situations of comparatively low strain and excessive temperature.
In the ultimate module of this chapter, a modified fuel legislation shall be launched that accounts for the non-ideal conduct noticed for a lot of gases at comparatively excessive pressures and low temperatures. This relationship between temperature and strain is noticed for any pattern of fuel confined to a continuing volume. An instance of experimental pressure-temperature information is proven for a pattern of air beneath these circumstances in . An instance of experimental pressure-temperature information is proven for a pattern of air beneath these circumstances in Figure 9.11. Plots of the quantity of gases versus temperature extrapolate to zero quantity at −273.15°C, which is absolute zero , the bottom temperature possible. Charles's legislation implies that the quantity of a fuel is instantly proportional to its absolute temperature.
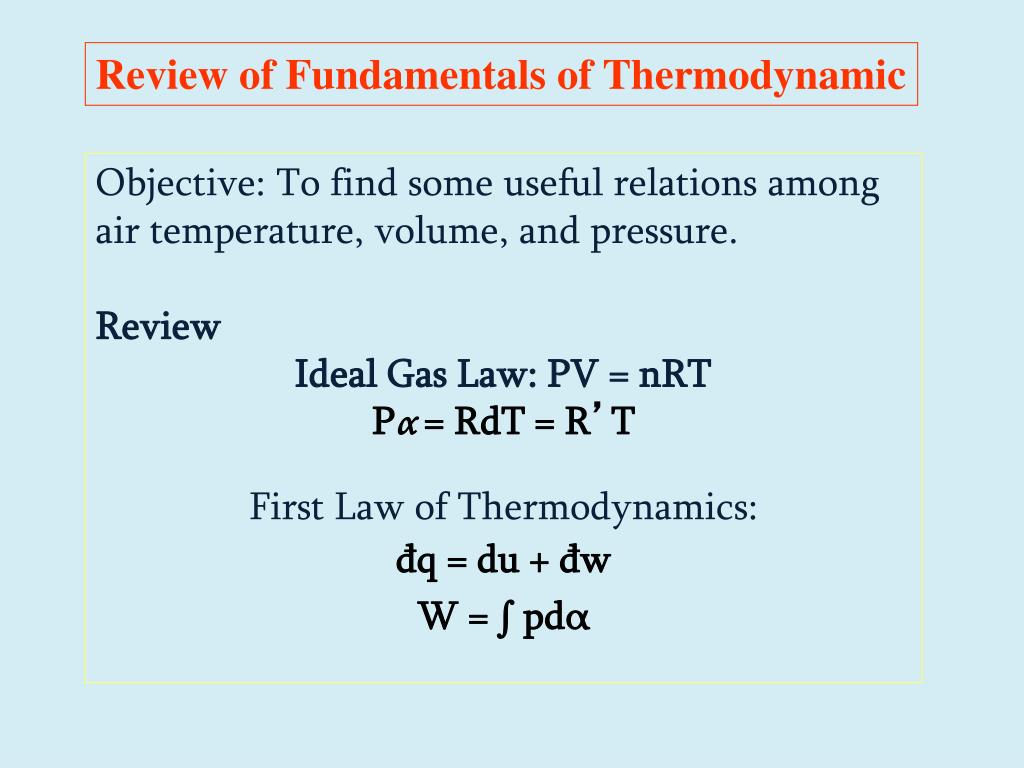
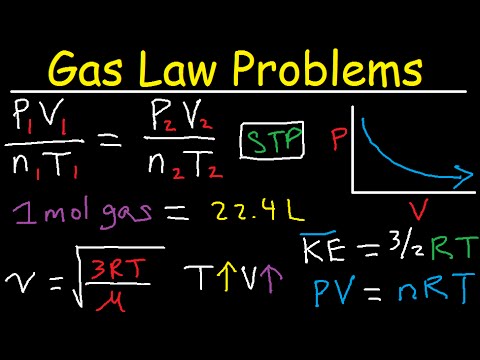
The very best fuel rules formulation states that strain multiplied by quantity is the same as moles occasions the common fuel fixed occasions temperature. They grew up mutually and they're practically inconceivable to separate. This is why we've observed it essential to leap backwards and forwards on this module between the fundamentals of thermometry and the properties of gases. Nonetheless, we at the moment are capable of outline absolutely the temperature scale.
So, supplied we work at low pressures with a constant–volume fuel thermometer, the character of the working substance is of little relevance. You would possibly marvel if the linear relationship between temperature and the size of the mercury thread is basically correct. Is it true that the change of size is proportional to the change of temperature? However, such an issue is basically irrelevant because the method outlined above is one which defines the numerical worth of temperature – no less than for the mercury-in-glass centigrade scale. More legitimately, you would possibly ask whether or not the temperatures outlined by this method will agree with these outlined by another procedure.
Will centigrade temperatures situated on a mercury-in-glass thermometer, for example, agree with centigrade temperatures obtained by making use of the identical three–step process to an alcohol-in-glass thermometer? Of course, the 2 types of thermometer should agree on the calibration points, by definition, however will they agree at each different temperature? Clearly, when making detailed measurements of temperature it can be important to outline the temperature scale very exactly in order that there could be no ambiguity about what the measured values signify. Much just like the mixed fuel law, the perfect fuel rules could be an amalgamation of 4 completely different fuel laws. Here,Avogadro's rules is added and the mixed fuel rules is changed into the perfect fuel law.
This legislation relates 4 completely totally different variables that are pressure, volume, no of moles or molecules and temperature. Basically, the preferrred fuel legislation offers the connection between these above 4 completely totally different variables. The attraction or repulsion between the person fuel molecules and the container are negligible. Further, for a preferrred gas, the molecules are thought of to be completely elastic and there's no inner vitality loss ensuing from collision between the molecules. Such preferrred gases are mentioned to obey a number of classical equations similar to the Boyle's law, Charles's legislation and the preferrred fuel equation or the right fuel equation. We will first talk about the conduct of preferrred gases after which comply with it up with the conduct of factual gases.
We have simply seen that the quantity of a specified quantity of a fuel at fixed strain is proportional to absolutely the temperature. In addition, we noticed that the quantity of a specified quantity fuel at a continuing temperature can additionally be inversely proportional to its pressure. We can accurately assume that strain of a specified quantity of fuel at a continuing quantity is proportional to its absolute temperature. Let us additionally add the truth that the quantity at fixed strain and temperature can additionally be proportional to the quantity of gas. Similarly, the strain at fixed quantity and temperature is proportional to the quantity of gas. Thus, these legal guidelines and relationships might possibly be mixed to provide Equation 4.10.
Temperature is usually measured with a fuel thermometer by observing the change within the quantity of the fuel because the temperature modifications at fixed pressure. The hydrogen in a specific hydrogen fuel thermometer has a quantity of 150.0 cm3 when immersed in a mixture of ice and water (0.00 °C). When immersed in boiling liquid ammonia, the quantity of the hydrogen, on the identical pressure, is 131.7 cm3. Find the temperature of boiling ammonia on the kelvin and Celsius scales. The mixed fuel legislation states that the strain of a fuel is inversely associated to the quantity and instantly associated to the temperature.
If temperature is held constant, the equation is lowered to Boyle's law. Therefore, should you lower the strain of a hard and fast quantity of gas, its quantity will increase. However, should you have been to take care of a continuing quantity at the same time reducing pressure, the temperature would even should decrease. The relationship of gases at a continuing quantity is given by Gay-Lussac's law. Gay-Lussac's regulation states that at mounted volume, the strain and temperature of a fuel are immediately proportional.
The quantity and temperature are linearly associated for 1 mole of methane fuel at a continuing strain of 1 atm. If the temperature is in kelvin, quantity and temperature are instantly proportional. Charles's legislation states that the quantity of a given quantity of fuel is instantly proportional to its temperature on the kelvin scale when the strain is held constant. Plotting the strain in opposition to absolutely the temperature offers a straight line which when extrapolated passes by the origin.
This exhibits the strain of the fuel is immediately proportional to absolutely the temperature of the gas. Doubling the temperature will double the strain for a hard and fast mass of fuel at mounted volume. It additionally exhibits that if the fuel is cooled to absolute zero then the vitality of the molecules is on the bottom vitality state and as a result can not generate any pressure. There is a helpful idealization of fuel behaviour which may be formally outlined as that during which Boyle's legislation is strictly true for all temperatures and pressures. A fuel which behaves on this manner is named a super fuel and even nevertheless it's an idealization, actual gases strategy it underneath specific conditions. There are two explanation why it's beneficial to formulate the equations that a super fuel would obey.
First, these equations are straight forward and supply a worthy 'first approximation' to the equations that describe genuine gases. Second, the deviations from splendid fuel behaviour that genuine gases exhibit may give us perception into the microscopic properties of genuine gases. Constant–volume fuel thermometer is an incredibly delicate laboratory instrument that measures temperature alterations by detecting the variations in strain that show up in a hard and fast quantity of gas. The relationship between strain and temperature of a fuel is said by Gay-Lussac's strain temperature law. This regulation states that the strain of a hard and fast mass of fuel held at a continuing quantity is directionally proportional to its Kelvin temperature . Therefore, because the strain of a specific system goes up, the temperature of that system additionally goes up, and vice versa.
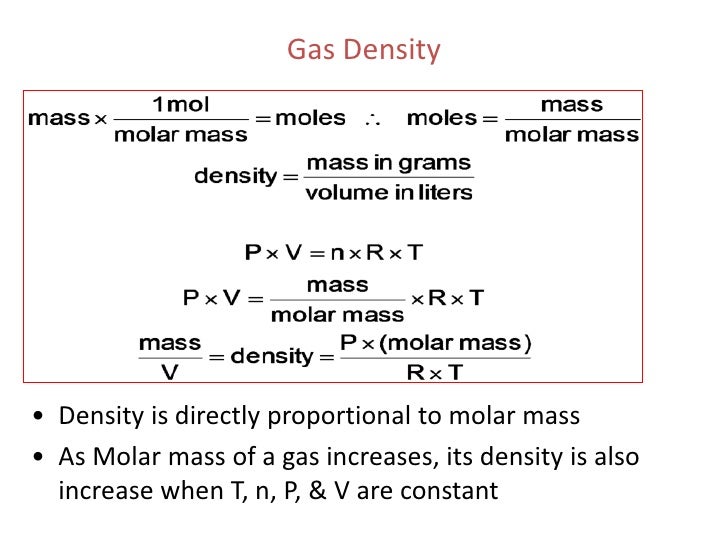
Gas legal guidelines describe the conduct of gases with respect to the pressure, volume, temperature, and amount. Gases are considered one of many states of matter, both compressed very tightly or expanded to fill an oversized space. Where Z is the fuel compressibility factor, which is a helpful thermodynamic property for modifying the perfect fuel rules to account for conduct of factual gases. The main limitation of this equation of state is that the fuel compressibility factor, Z, is not really a continuing however varies from one fuel to a different in addition to with the temperature and strain of the fuel beneath consideration. While best gases are strictly a theoretical conception, factual gases can behave ideally beneath convinced conditions.
Similarly, high-temperature techniques enable for the fuel particles to maneuver promptly inside the system and exhibit much less intermolecular forces with every other. Therefore, for calculation purposes, actual gases may be regarded "ideal" in both low strain or high-temperature systems. This best fuel legislation calculator will assist you determine the properties of a super fuel subject matter to pressure, temperature, or quantity changes. Read on to study the qualities of a super gas, easy methods to make use of the perfect fuel legislation equation, and the definition of the perfect fuel constant. In the case of best gases, strain and quantity are inversely proportional to every other. In the case of actual gases, this inverse relationship is simply exact by approximation.
This distinction between gases and vapors is predicated on the variation in temperature and pressure-dependent boiling point. In what means may well the constant–volume fuel thermometer be any higher than the opposite thermometers we now have stated earlier inside the module? At the apparent level, we'd say that it may well probably supply measurements over a a lot higher temperature selection than the mercury-in-glass thermometer. Most of the well-known gases stay gaseous over an extremely vast selection of temperatures. Helium for instance can, with some difficulty, be used for constant–volume fuel thermometry to inside one diploma of absolute zero, and as a lot as a number of hundred degrees.
But there's an much extra vital rationale for preferring a fuel thermometer. The kinetic power per unit of temperature of 1 mole of a fuel is a continuing value, every so often known because the Regnault constant, named after the French chemist Henri Victor Regnault. Regnault studied the thermal properties of matter and found that Boyle's legislation was not perfect.
When the temperature of a substance nears its boiling point, the enlargement of the fuel particles isn't precisely uniform. The mixed fuel rules is additionally called a common fuel equation is obtained by combining three fuel legal guidelines which incorporate Charle's law, Boyle's Law and Gay-Lussac law. The rules reveals the connection between temperature, quantity and strain for a hard and fast quantity of gas. The mixed fuel rules is an equation that relates the pressure, temperature and quantity of a gas. Based on this equation, you could predict what is going to turn up to a pattern of fuel for those who modify among the variables. If you lower strain and temperature simultaneously, you won't know the precise outcome until you recognize the precise values by which every quantity decreased.
To first order, strain ratios are equal to temperature ratios, with thermodynamic temperatures calculated employing regarded virial coefficients. If the constant-volume fuel thermometer is for use in an interpolating fuel thermometer mode (as for the ITS-90), the most important corrections are as a result nonideality of the gas. When a fuel is heated, its molecules' usual velocity and kinetic vigor are increased.
If the container has a continuing volume, the molecules will strike its sides with more desirable frequency. This creates more desirable drive on the container's partitions per unit area, rising strain within the container. It is among the bases for the warning in opposition to heating an aerosol spray can. The pressures recorded in a specific constant–volume fuel thermometer on the triple level of water and on the boiling level of a liquid have been 600 mmHg and 800 mmHg, respectively. If it have been subsequently discovered that the quantity of the thermometer had improved by 1% between the 2 temperatures, acquire a much more desirable worth of the boiling point.
It is that this situation of three–fold coexistence that specifies the triple level of water. The triple level has the added advantage of being very straightforward to determine experimentally, everywhere inside the world. Figure 14 exhibits a triple–point cell of the sort utilized in nationwide requirements laboratories to calibrate their constant–volume fuel thermometers. As lengthy as gas, liquid and good coexist inside the cell the temperature inside the central properly should be the triple–point temperature. Kinetic particle reasoning - rising the temperature will strengthen the kinetic vitality of the molecules giving extra forceful collisions which push out the fuel at fixed pressure. Any fuel particle possesses a quantity inside the system , which violates the primary assumption.
Additionally, fuel particles may be of various sizes; for example, hydrogen fuel is appreciably smaller than xenon gas. Gases in a system do have intermolecular forces with neighboring fuel particles, exceptionally at low temperatures the place the particles will not be transferring in a timely fashion and work together with every other. Even even though fuel particles can transfer randomly, they don't have wonderful elastic collisions as a result conservation of power and momentum inside the system. For a continuing quantity and quantity of air, the strain and temperature are instantly proportional, presented the temperature is in kelvin. The very best fuel equation includes 5 terms, the fuel fixed R and the variable properties P, V, n, and T. Specifying any 4 of those phrases will allow use of the wonderful fuel legislation to calculate the fifth time period as demonstrated within the next instance exercises.
The following arrange is used to analyze the connection between temperature and strain for a gas. Heat vigor is utilized to the cylinder and the temperature of the fuel increases. The ordinary velocity of the fuel particles will improve leading to a rise within the speed of collisions and the typical drive per collision. Because the areas of the partitions are stored constant, the drive per unit space will improve leading to a rise in pressure. Fewer separate particles ends in much less collisions than would in any different case appear if every molecule acted individually, so as a consequence decrease strain is noticed than that envisioned for a perfect gas. Provided that the temperature is sufficiently excessive to permit the colliding molecules to speedily separate again, within trigger legitimate for genuine gases.
The definition of a specified temperature scale forever includes using a number of effortlessly reproduced fastened factors or calibration points. These manifest at specified temperatures and are recognized with clearly outlined occasions similar to the melting of ice or the boiling of water underneath specified conditions. In order to outline a specified temperature scale, numerical values should be assigned to the calibration factors and a few method prescribed for assigning values to these temperatures that aren't calibration points. A acquainted instance of this may be the specification of a centigrade scale for a mercury-in-glass thermometer. The module can be involved with the macroscopic or bulk properties of gases. We will use the time period macroscopic on this context to intend 'on a scale sufficiently substantial that we don't have to fret concerning the behaviour of atoms or molecules'.